FDA approved Botox Cosmetic
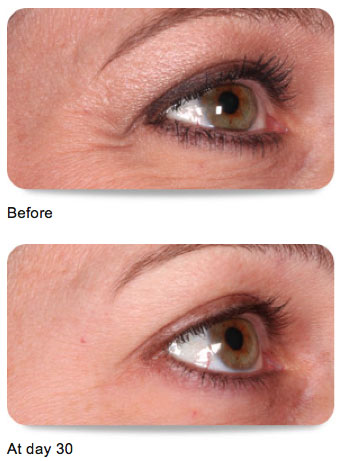 The FDA recently approved the use of Botox Cosmetic to improve the appearance of crow’s feet lines. (Read news release here)
The FDA recently approved the use of Botox Cosmetic to improve the appearance of crow’s feet lines. (Read news release here)
BOTOX® Cosmetic (onabotulinumtoxinA) Important Information.
Indications
Glabellar Lines
BOTOX® Cosmetic (onabotulinumtoxinA) for injection is indicated for the temporary improvement in the appearance of moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity in adult patients.
Lateral Canthal Lines
BOTOX® Cosmetic is indicated for the temporary improvement in the appearance of moderate to severe lateral canthal lines associated with orbicularis oculi activity in adult patients.
Contact us for a consultation at our Huntsville office where you can ask Dr. Miles any questions about BOTOX® for a better understanding.
WARNINGS AND PRECAUTIONS
Lack of Interchangeability between Botulinum Toxin Products
The potency Units of BOTOX® Cosmetic are specific to the preparation and assay method utilized. They are not interchangeable with other preparations of botulinum toxin products and, therefore, units of biological activity of BOTOX® Cosmetic cannot be compared to nor converted into units of any other botulinum toxin products assessed with any other specific assay method.
Spread of Toxin Effect
Please refer to Boxed Warning for Distant Spread of Toxin Effect.
No definitive serious adverse event reports of distant spread of toxin effect associated with dermatologic use of BOTOX® Cosmetic at the labeled dose of 20 Units (for glabellar lines), 24 Units (for lateral canthal lines), 44 Units (for simultaneous treatment of lateral canthal lines and glabellar lines) have been reported.
Injections In or Near Vulnerable Anatomic Structures
Care should be taken when injecting in or near vulnerable anatomic structures. Serious adverse events including fatal outcomes have been reported in patients who had received BOTOX® injected directly into salivary glands, the oro-lingual-pharyngeal region, esophagus and stomach. Safety and effectiveness have not been established for indications pertaining to these injection sites. Some patients had pre-existing dysphagia or significant debility. Pneumothorax associated with injection procedure has been reported following the administration of BOTOX® near the thorax. Caution is warranted when injecting in proximity to the lung, particularly the apices.
Hypersensitivity Reactions
Serious and/or immediate hypersensitivity reactions have been reported. These reactions include anaphylaxis, serum sickness, urticaria, soft-tissue edema, and dyspnea. If such reactions occur, further injection of
BOTOX® Cosmetic should be discontinued and appropriate medical therapy immediately instituted. One fatal case of anaphylaxis has been reported in which lidocaine was used as the diluent and, consequently, the causal agent cannot be reliably determined.
Cardiovascular System
There have been reports following administration of BOTOX® of adverse events involving the cardiovascular system, including arrhythmia and myocardial infarction, some with fatal outcomes. Some of these patients had risk factors including pre-existing cardiovascular disease. Use caution when administering to patients with pre-existing cardiovascular disease.
Pre-existing Neuromuscular Disorders
Individuals with peripheral motor neuropathic diseases, amyotrophic lateral sclerosis, or neuromuscular junctional disorders (eg, myasthenia gravis or Lambert-Eaton syndrome) should be monitored particularly closely when given botulinum toxin. Patients with neuromuscular disorders may be at increased risk of clinically significant effects including severe dysphagia and respiratory compromise from typical doses of BOTOX® Cosmetic.
Pre-existing Conditions at the Injection Site
Caution should be used when BOTOX® Cosmetic treatment is used in the presence of inflammation at the proposed injection site(s) or when excessive weakness or atrophy is present in the target muscle(s).
Human Albumin and Transmission of Viral Diseases
This product contains albumin, a derivative of human blood. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of viral diseases. A theoretical risk for transmission of Creutzfeldt-Jakob disease (CJD) also is considered extremely remote. No cases of transmission of viral diseases or CJD have ever been identified for albumin.


